Injectafer’s Life-Threatening Side Effect: Are You at Risk?
If you’ve taken Injectafer and suffered from hypophosphatemia, don’t delay. Contact our experienced defective drug attorneys to see if you have grounds for a lawsuit.
Injectafer Hypophosphatemia Lawsuit: Should You File?
Injectafer (ferric carboxymaltose) Lawsuits Claim Life-Threatening Side Effects Discovered, Withheld From Marketing
If you or someone you love has been prescribed Injectafer as treatment for iron deficiency anemia, you may be at risk for a potentially life-threatening side effect. Since the drug was approved for sale in 2013, numerous studies have shown that patients who are administered Injectafer are at much higher risk of developing hypophosphatemia.
We believe this risk is unnecessary and should have been disclosed much sooner. If you or a loved one has suffered serious Injectafer side effects – specifically hypophosphatemia – please contact us.
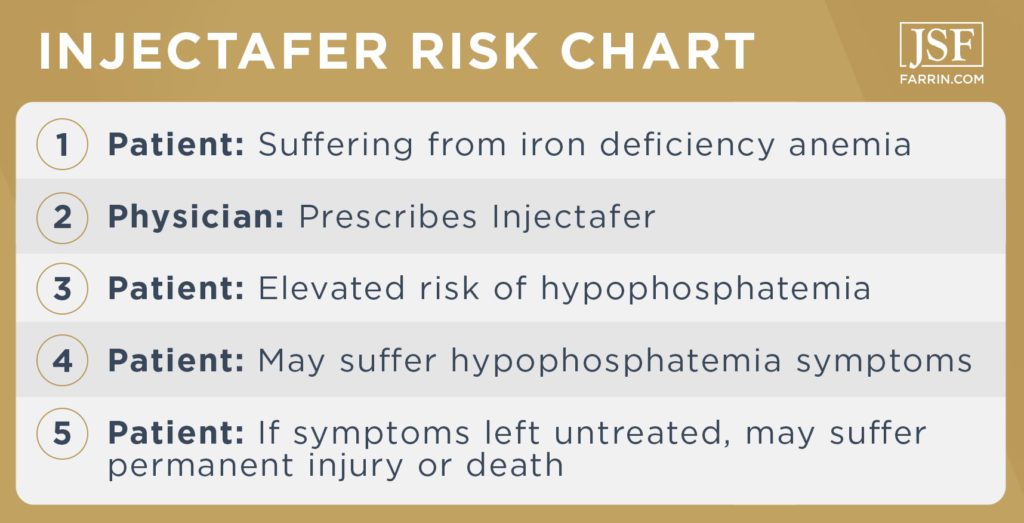
Injectafer Risk Chart and Key Terms
To understand the seriousness of the situation, you want to know a few key definitions and terms.
Injectafer
This is an injectable treatment for people with iron deficiency anemia and chronic kidney disease who are not on dialysis. The drug is also known as ferric carboxymaltose (FCM). It is intended to introduce elemental iron into the blood to help the body produce red blood cells which carry oxygen. Injectafer iron infusions are prescribed to patients for whom oral iron supplements have proven ineffective. It is, however, not the only option. Multiple studies have shown an increased risk of hypophosphatemia in users of Injectafer.
Iron Deficiency Anemia
A common form of anemia – meaning the body lacks sufficient healthy red blood cells – caused by insufficient iron. This is the condition that Injectafer was developed to treat, as iron deficiency anemia can cause organ damage/ischemia if left untreated in some patients. Symptoms include:
- Extreme fatigue
- Weakness
- Pale skin
- Chest pain, fast heartbeat or shortness of breath
- Headache, dizziness or lightheadedness
- Cold hands and feet
- Inflammation or soreness of your tongue
- Brittle nails
- Unusual cravings for non-nutritive substances, such as ice, dirt or starch
- Poor appetite, especially in infants and children with iron deficiency anemia
Hypophosphatemia
A condition characterized by low levels of phosphate in the blood. Phosphate is an electrolyte, and the body uses it for energy production and nerve function as well as bone health. Most of your body’s phosphates are stored in the bones.
Note that severe hypophosphatemia can be life threatening. The condition can lead to bone weakness and fractures, muscle damage, death of muscle tissue (rhabdomyolysis), breathing failure, red blood cell destruction (hemolytic anemia), and/or an irregular heart rhythm (arrhythmia).
If you or someone you love has been prescribed or taken Injectafer and suffered symptoms of hypophosphatemia, consult with an attorney who has experience dealing with dangerous drugs and their victims about a potential Injectafer lawsuit.
Injectafer Health Risks Shown in Multiple Studies
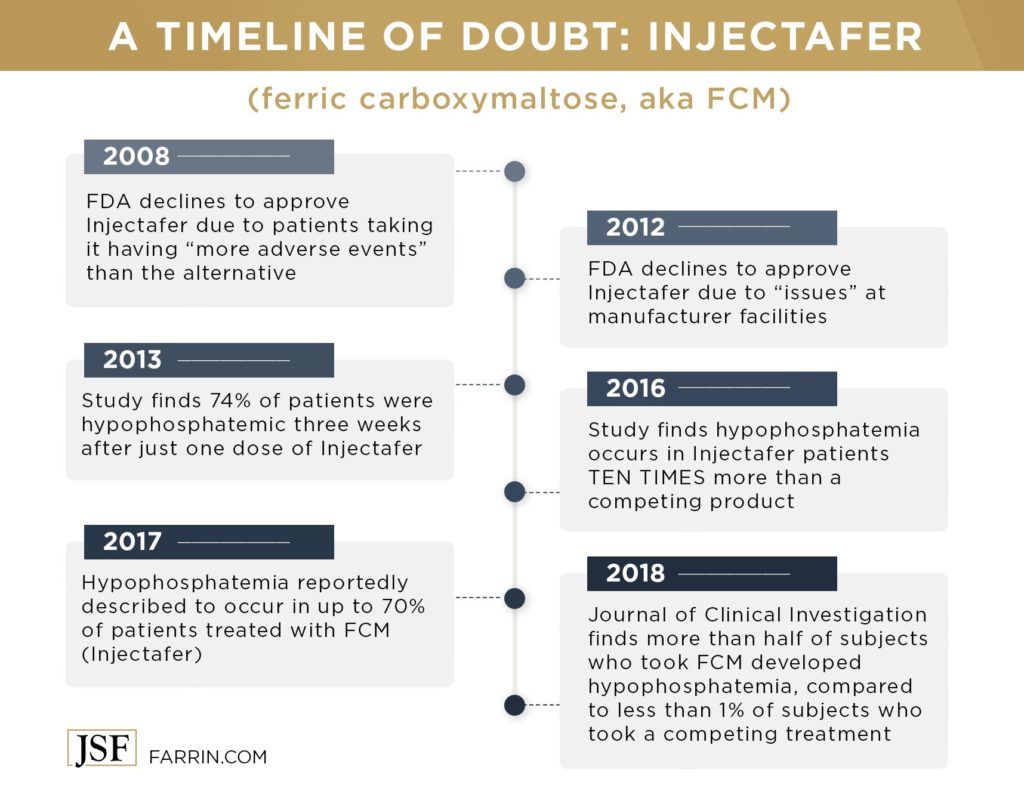
Since it was approved by the FDA for use in 2012, Injectafer (FCM) has been marketed with a list of possible side effects. Multiple subsequent clinical studies have shown the drug to have an “undisclosed” side effect.
- A 2013 study of 47 non-dialysis patients with chronic kidney disorder suffering from iron deficiency anemia showed an alarming increase in hypophosphatemia. The study observed these 47 patients who received a single 1000mg injection of FCM prior to the administration of the drug, and then three weeks after. Of the 47 subjects, 35 were classified as hypophosphatemic after three weeks.
- In 2016, a study compared FCM to iron isomaltoside 1000 (IIM). Both are iron carbohydrate complexes that treat iron deficiency anemia. The study included 81 patients. Those who were administered FCM were found to be at more than ten times greater risk for hypophosphatemia (45%) than those who took IIM (4%). Severe hypophosphatemia occurred only in patients taking FCM (32.7%).
- A 2017 response to a prior study indicated that “hypophosphatemia … has indeed been described to occur in up to 70% of patients treated with [FCM].”
- The Journal of Clinical Investigation published the results of a randomized, double-blind, controlled study of adults with iron deficiency anemia in 2018. From February 2016 to January 2017, researchers compared rates of hypophosphatemia in response to a single FDA-approved course of FCM or ferumoxytol. The results were astonishing – more than half (50.8%) of patients treated with FCM developed hypophosphatemia versus less than 1% (0.9%) of those treated with ferumoxytol. Furthermore, the condition persisted in more than 29% of the FCM patients through the end of the five-week study period.
Injectafer and the FDA: Denials, Failures, and Misleading Marketing
The manufacturer tried three different times to get approvals from the FDA to market Injectafer, and was denied the first two times in 2008 and 2012. The first rejection was due to the fact that patients taking the drug had “more adverse events” than those taking oral iron supplements. In 2012, Injectafer iron infusion products were denied because the FDA’s inspection of one of the company’s manufacturing facilities revealed “issues.”
In January of 2015, the FDA issued manufacturer Luitpold Pharmaceuticals a warning about false or misleading marketing for Injectafer – and this warning did not even stem from the crowing evidence of hypophosphatemia risk. The warning cited lack of adequate directions for use, minimization of risk, omission of material fact, and misleading claims.
Faced with the mounting evidence of a problem, the maker of Injectafer, the manufacturer, finally appended hypophosphatemia to the drug’s list of possible side effects in February 2020 – about eight years too late. As of October 2020, the FDA has not yet issued an Injectafer recall or any warning to the public regarding the apparent danger of using the product.
Note: Luitpold Pharmaceuticals is a wholly owned subsidiary of Daiichi Sankyo.
Potential Long-Term Side Effects of Hypophosphatemia Resulting from Injectafer Use
While hypophosphatemia is not solely linked to the use of Injectafer (FCM), the research-proven increase in its occurrence following use of Injectafer should cause great concern for its users and their physicians.
Hypophosphatemia has many symptoms, and they may not seem related. These include:
- Muscle weakness
- Fatigue
- Bone pain
- Bone fractures
- Appetite loss
- Irritability
- Numbness
- Confusion
When hypophosphatemia becomes severe, the effects can be devastating and even fatal. Anorexia, muscle weakness, and osteomalacia (softening of the bones) can occur in severe chronic phosphate depletion. Serious neuromuscular disturbances may occur, including progressive encephalopathy (deterioration of the brain), seizures, coma, and death. The muscle weakness of profound hypophosphatemia may be accompanied by rhabdomyolysis (a breakdown of muscle tissue that releases a damaging protein into the blood).
If You or a Loved One Has Taken Injectafer and Suffered From Hypophosphatemia, Seek Help Immediately
First, consult your physician. Then, contact the experienced defective drug attorneys at the Law Offices of James Scott Farrin to see if you have grounds for a potential Injectafer lawsuit. Fight for your rights, and against negligent drug manufacturers whose carelessness does you harm. Let us help you try to hold them accountable.
Call us right away at 1-866-900-7078 for a free case evaluation, or simply contact us online. We’re available 24/7 to assist you, so there’s no reason to delay. In cases like these, time is of the essence. The sooner you call, the sooner we can evaluate the strength of your claim! Don’t just let drug companies get away with marketing flawed products. Tell them you mean business.
Text Us
The Law Offices of James Scott Farrin consults with a national network of attorneys on product liability, defective drugs, and defective products cases in an attempt to provide the best representation we can for our clients. Depending on the details of your case, our firm will likely refer your matter to another law firm with which we associate. We will only do this if we believe it is in your best interests and if you agree.