Surgical Staplers: Has Its History of Failure Affected You?
If your surgical stapler was defective, you may be experiencing catastrophic consequences. Are you entitled to compensation for your suffering?
Looking to File a Surgical Stapler Complications Lawsuit?
Surgical Staplers: Recalls and Dire Warnings After Injuries, Death Reported
In March 2019, the FDA reported more than 41,000 reports of stapler incidents dating back to 2011, finding 366 deaths, more than 9,000 serious injuries, and more than 32,000 malfunctions. It has issued a Class I recall, even as reports of injuries continue to appear.
What Are Surgical Staplers and How Do They Work?
Using staples in surgery is not a new method. Surgical staplers were introduced in 1908 by Hungarian surgeon Humer Hultl. They were brought to the U.S. more than fifty years later. The first American company to produce them was United States Surgical Corporation in 1964. Since then, other manufacturers have entered the business, including Johnson & Johnson under the Ethicon brand, Covidien, and Medtronic (which merged with Covidien in 2014).
They are designed to suture, connect, or seal tissue using metal staples that are formed by the stapler where the surgeon desires. Essentially, there are two classes of staplers – disposable and non-disposable. There are three styles of staples that can be created by different staplers, depending on the type of connection required by the procedure. Those are straight, curved, or circular. The staples are made of metal, either stainless steel or titanium, and have to be removed at some point. Plastic staples are also used, and there are staples made of polylactide-polyglycolide copolymer that are designed to be absorbed into the body.
The signs of a misfired or malformed staple may not occur for several days after the procedure. They’re typically used in procedures such as:
- Gastrointestinal Surgery
- Gastric Bypass Surgery (Stomach Stapling)
- Bowel Surgery
- Appendectomy
- Lung Surgery
- Thoracic Surgery (Neck Related)
- Cardiovascular Surgery (Heart Related)
How Do Ethicon Staplers Fail?
The Ethicon Flex Endopath models in the latest recall are the disposable type, intended for use in one single operation and use metal staples. In their reasoning for the Class I recall – the most urgent type of recall, as it involves the risk of patient harm or even death – the FDA stated that the recalled devices contain an out-of-specification part within the jaw of the device that can cause staples to be malformed.
Malformed staples, in turn, can lead to serious consequences for patients, including:
- Prolonged surgeries
- Postoperative connection (anastomotic) leaks
- Hemorrhages
- Hemorrhagic shock
- Additional surgeries to correct or intervene
- Death
There have been seven injuries reported and one death reported leading up to this latest recall. It is the second recall of Ethicon brand staplers in the last two years. The current recall affects 8,256 of the following models and batch numbers distributed between August 1 and September 26, 2019:
- Flex 60 Endopath Stapler, Articulating Endoscopic Linear Cutter, product code EC60A, lot Nos. T9408M and T94A9Z.
- Flex 60 Powered Plus Compact Articulating Endoscopic Linear Cutter, product code PCEE60A, lot Nos. T93Z9Y and T9411A.
- Flex 60 Powered Plus Articulating Endoscopic Linear Cutter, 44cm Shaft Length, product code PLEE60A, lot Nos. T93X95; T93Z75; T93Z2W; T9413Z; T93Z1G; T93Y4M; T94045; T94117; T93X17; T94087; T93Z2X; T9405L; and T94253
- Flex 60 Powered Plus Articulating Endoscopic Linear Cutter, 34cm Shaft Length, product code PSEE60A, lot Nos. T93Z5W; T93Z5X; T9405V; T9405W; T93Z3F; T9401L; T93Y8X; T94008; T9400D; T93Z5R.
A History of Harm: Previous Ethicon Recall
The October 2019 recall from the FDA is disturbing, but it is sadly not new for Ethicon. In May 2019, the FDA issued a Class I recall for several of their Curved Intraluminal Staplers. Investigation after complaints revealed that some products contained uncut washers and a firing issue that caused malformed staples.
This was attributed to a change in the manufacturing process that was introduced in March of 2018 and continued through March of 2019. This prompted a recall of 92,496 of the following products distributed between March 15, 2018, and March 8, 2019:
- Endo-Surgery Curved Intraluminal Stapler with Adjustable Height Staples
- Endo-Surgery Endoscopic Curved Intraluminal Stapler with Adjustable Height Staples
- Product Codes: CDH21A, CDH25A, CDH29A, CDH33A, ECS21A, ECS25A, ECS29A, ECS33A
In that recall, the FDA recommended that surgeons use manual staplers from other manufacturers, hand-sew closures, use minimally invasive techniques where possible, or delay surgeries where the staplers’ use was necessary. The staplers had been used in a variety of operations on patients, and their failures produced a wide range of serious injuries and side effects, including:
- Bleeding
- Sepsis
- Bacterial infection
- Need for lifelong ostomy “bag”
- Lifelong nutritional and digestive issues
- Anastomotic leaks
- Need for additional surgeries or closures
- Need for antibiotics and/or additional imaging studies
Covidien/Medtronic Surgical Staplers: Too Many Adverse Events
Ethicon is not the only device manufacturer facing intense scrutiny over its surgical staplers. According to multiple lawsuits, medical device maker Medtronic, which merged with Covidien in 2015, allegedly knowingly sold defective products while hiding the serious risks from doctors and patients. Some patients say they were injured when Medtronic staplers malfunctioned during their surgical procedures. The staplers would:
- Leave holes but no staple; or
- Not properly embed the staple
These open wounds led to:
- Severe infections; and/or
- Heart problems; and/or
- Corrective surgeries
Total additional medical expenses were in the hundreds of thousands, never mind the pain and suffering.
The Hidden Truth: Buried Safety Reports
Surgical staplers are a multi-billion dollar industry, and Covidien/Medtronic is one of its top players. In addition to designing a defective product and allegedly hiding information it knew about the significant risks, Medtronic is alleged to have used a “loophole” in FDA reporting to keep the public in the dark about the clear and ongoing danger of the staplers and staples.
While the company reported adverse events like serious injuries and death – as required by federal law – it buried many of the reports in non-public files known as Alternative Summary Reports. Increased media attention on the ASR program starting in 2016 forced the FDA to reveal some startling new information on the dangers of surgical staplers.
The FDA reviewed millions of reports in the ASR system. These reports included tens of thousands from Medtronic and Covidien regarding their surgical staplers. Before the review, 53,720 problems with all models of staples and staplers had been shared publicly. After the review, over 56 thousand reports of surgical stapler problems were added to the tally.
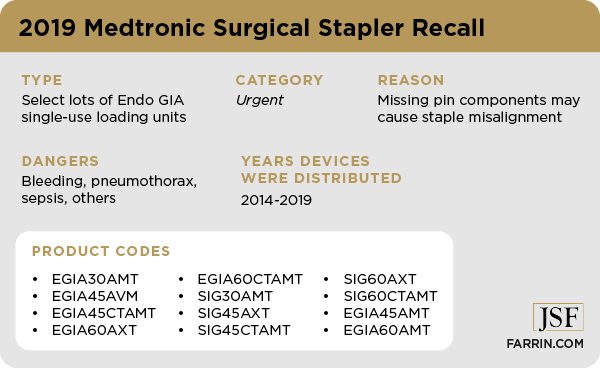
The method Medtronic used for notification of adverse events was so controversial that it was eliminated entirely in 2019. 2019 was also the year Medtronic had to recall some of its surgical staplers because they were potentially missing necessary parts. The missing pins were designed to prevent problems with the stapler, including complications like anastomotic leaks.
Tightening FDA Approval Requirements – Too Little Too Late?
ECRI, an independent, non-profit patient safety organization, placed surgical staplers at the top of its list of health technology hazards in October 2019. This after the FDA proposed, in April 2019, reclassifying surgical staplers from Class I to Class II.
Class I devices are not heavily tested or regulated and do not undergo premarket review, and include things like cotton swabs and crutches. Class II devices must undergo a premarket review by the FDA before being approved for use.
Manufacturers claim that they treat staplers as Class II devices, even though the FDA does not perform premarket reviews on them. The FDA has already classified implantable surgical staples as the more regulated and tested Class II devices. Oddly, the staplers themselves have remained Class I.
However, the move toward additional regulation came after a review of the FDA’s internal database and the alternative medical reporting system for instances occurring between January 1, 2011, and December 31, 2018. The agency has noted that its reliance on manufacturers, physicians, and other voluntary reporters to record malfunctions is “imperfect.”
The databases contained records on approximately 110,000 malfunctions, and 422 deaths. As previously noted, surgical staplers have been in use for decades. For this many malfunctions to be reported – and perhaps underreported – in just eight years is mind-boggling.
If You Were Hurt or Suffered Complications Due to Defective Surgical Staplers, We May Be Able to Help
Whether your procedure was routine or emergency, you have every right to expect that the products used to treat you will function properly. When those products fail, the consequences can be catastrophic. Suddenly, a procedure that was supposed to bring you relief only brings you grief.
If you or a loved one has suffered or is suffering the effects of a malformed surgical staple, call the Law Offices of James Scott Farrin at 1-866-900-7078 for a free case evaluation, or contact us online.
The Law Offices of James Scott Farrin consults with a national network of attorneys on product liability, defective drugs, and defective products cases in an attempt to provide the best representation we can for our clients. Depending on the details of your case, our firm will likely refer your matter to another law firm with which we associate. We will only do this if we believe it is in your best interests and if you agree.