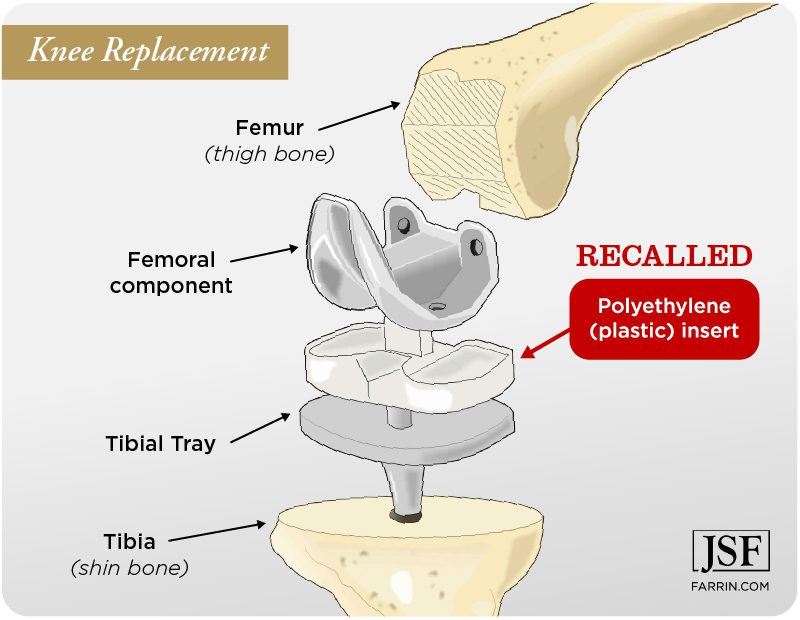
Are you affected by the Exactech recall?
More than 100,000 Americans have Exactech replacement joints that may have suffered premature wear due to faulty packaging. While the company issued a recall, it’s not that easy for many patients.
Affected by the Exactech Replacement Joint Recall?
Thousands of people in the United States have received artificial ankles, knees, and hips made by Exactech. In early 2022, the company found the faulty packaging may have allowed oxygen into some devices. Exposure to oxygen can damage the polyethylene liners in the joints, leading to premature or accelerated wear.
In response, the company issued a recall on several models of its replacement joints dating back to 2004. The number of devices recalled, according to the company, is nearly half a million. How did this happen? What did the company know? Has it been completely transparent? Historically, there are some worrying signs.
Current Exactech Recall Details
The current recall covers some 147,732 devices in the United States and began as early as August 2021. The FDA classifies it as a Class II recall, which involves:
“temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote”
While technically accurate, that description fails to factor in three critical issues.
- Patients must be healthy enough to undergo the revision surgery.
- Debris from the failing implant can destroy surrounding tissue, causing pain, infection, and other side effects.
- Revision surgery is not inexpensive and can require lengthy recovery times.
Which Exactech products are recalled and why?
Exactech’s OPTETRAK®, OPTETRAK Logic®, TRULIANT®, and VANTAGE® implants for knees and ankles are all part of the recall due to the possible degradation of the tibial inserts and liner components. According to the company, faulty vacuum packaging for the polyethylene inserts may have allowed oxygen to contact the inserts. The resulting oxidation can degrade the material, leading to premature wear and failure.
What are the symptoms of a failing Exactech replacement joint?
Talk to your doctor or orthopedic surgeon as soon as possible if you’re experiencing any of these symptoms of replacement joint failure:
- New or worsening swelling of the joint
- Pain while walking
- Grinding feelings or noise, or clicking or other unusual noises in the joint
- Instability due to the joint
- Inability to bear weight on the joint
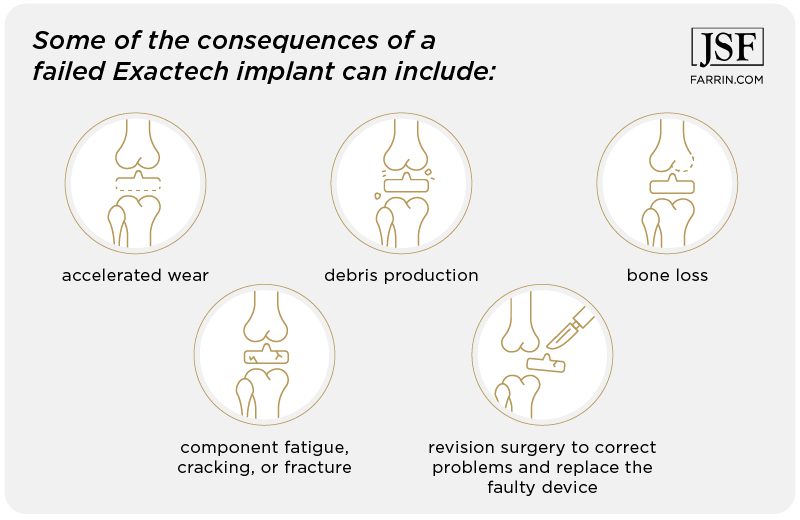
These may be signs of premature failure in your Exactech replacement joint and require revision surgery. Talk to your doctor, then call us. Some of the consequences of a failed Exactech implant can include:
- accelerated wear
- debris production
- bone loss
- component fatigue, cracking, or fracture
- revision surgery to correct problems and replace the faulty device
Exactech in the News Again?
In recall cases, many consumers may base their perceptions – and perhaps their course of action – on the company involved. Some might decline to seek compensation unless incredibly inconvenienced because they see the problem as an “honest mistake.”
History tells us, however, that some companies aren’t always on the level with consumers. There are a few things you may want to know if you’re considering letting Exactech off the hook. While unrelated to this specific recall, the government has sued the company for questionable business practices regarding its implants.
Another possible Exactech recall?
Exactech recently informed surgeons that patients with its Connexion GXL hip implants might experience premature wear. It is phasing the product out. No recall has been issued, but lawsuits have been filed over the failures of the devices.
Higher failure rates in Exactech devices?
According to DrugWatch, Exactech’s OPTETREK knee replacements may have experienced higher revision rates – that is, replacement of the devices – since their introduction in the early 90s. Data from the UK, Australia, and New Zealand reveal rates as much as seven times higher than other devices. Reports on the FDA’s Manufacturer and User Facility Device Experience (MAUDE) appear to indicate a higher-than-average failure rate as well.
Whistleblowers accuse Exactech of illegal practices
Furthermore, the company has previously faced whistleblower lawsuits. One claimed it knowingly gave faulty devices to Medicare, Medicaid, and Department of Veterans Affairs patients. Another claims it offered “phony consulting deals” to silence surgeons who complained about alarming defects in its knee replacements.
What should Exactech implant recipients do now?
The first step would be to talk to your physician or orthopedic surgeon. They may want to perform tests or X-rays to evaluate the joint and locate any premature wear. You can also look up your device’s serial number to determine if it is part of the recall. Even if your device is not failing yet, you should contact us and allow our defective device attorneys to review your information. You may be entitled to compensation.
Exactech has hired a settlement adjusting company – they do not represent you
In addition to sending a form letter for orthopedic surgeons to send to patients, Exactech has retained Broadspire, a settlement adjustment firm. It is vital to understand that Broadspire does not represent your best interests. Exactech employs them.
Therefore, if you receive communication that directs you to their helpline or to file a claim, you should consult with a defective device attorney first. We can help with your compensation claim. While they may offer limited medical and out-of-pocket reimbursement costs, the price of a revision surgery could be much higher. Consider the following possible costs:
- Medical expenses, including deductibles, follow-up care, medication, and therapy
- Lost wages for time out of work, if applicable
- Pain and suffering through a procedure you shouldn’t have needed
- Loss of consort and enjoyment of life while undergoing and recovering from the procedure
- Possible permanent injury from the revision or the original failed implant
Contact Our Defective Device Attorneys First
We put your best interests first. A free, no-obligation case evaluation is just a phone call away – 1-866-900-7078. Or, if you prefer, you can chat with us or contact us online. Someone is available to speak with you 24/7, listen to your concerns, and help you. We don’t charge an attorney’s fee unless we collect on your behalf. Guaranteed.2
Don’t hesitate, and don’t suffer with a defective joint implant. Take action, and tell them you mean business!
The Law Offices of James Scott Farrin consults with a national network of attorneys on product liability, defective drugs, and defective products cases in an attempt to provide the best representation we can for our clients. Depending on the details of your case, our firm will likely refer your matter to another law firm with which we associate. We will only do this if we believe it is in your best interests and if you agree.